Naming Of Bigger Compounds
GOC of Class 11
Naming Of Bigger Compounds
Having learnt the names of simple compounds we are now in happy situation to proceed to name the bigger or complex compounds by IUPAC system. There are however more than 70 rules in the system which guide us to name the organic compounds but here we are required to know only six basic rules.
- Rule−1: Select the longest continuous carbon chain in a molecule whether it is in straight line (I) or not in straight line (II).
-
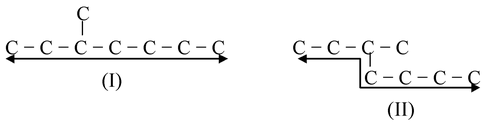
- Rule−2: If the compounds contains one or more longest continuous carbon chains then that chain is accepted which has the largest number of branches.
- Let us understand it through an example,
-
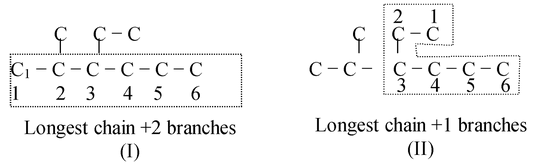
- The above two structural formulae are identical with, difference that if we view it in two ways (I) and (II). Both have the longest chains of 6 carbons atoms, which are numbered. In viewing as (I) we get two branches while viewed as (II) we get one branch.
- So, (I) is the correct longest chain.
- Rule−3: The rule states that after selecting the longest carbon chain, lowest position number is to be given to the position of substituent say, S. Let us have an example
-
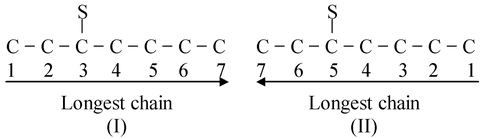
- In (I) 3 is the lower. Hence (I) is accepted and (II) is rejected. The positional number is also termed as LOCANT which means a number that locates the position of a substituent eg. Locant of S in (I)=3 and locant of S in (II)=5 due to incorrect numbering.
- Rule−4: This rule is applicable to those compounds which have more than one substituent(S). In such cases counting is done from that terminal of carbon chain which gives lesser value for the sum of locant values of substituents. Such as
-
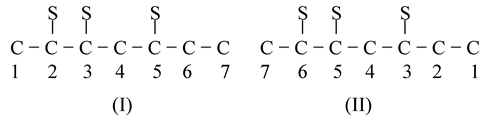
- 2 + 3 + 5 = 10 3 + 5 + 6 = 14
- The compounds are identical and after counting we get 7C−atoms. But after counting, the sum of the locants of S should be lowest. Hence (I) is accepted for naming the compound.
<
IUPAC names of compounds containing poly functional groups
| Functional groups | Formula | Name as substituent | Name as parent |
| Acid | −COOH | Carboxy |
Carboxylic or −oic
acid |
| Sulphonic acid | −SO3H | Sulpho | Sulphonic acid |
| Ester |
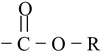 |
Alkoxycarbonyl | –oate |
| Anhydride |
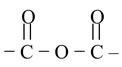 |
– | –oic anhydride |
| Acid halide | −COX | Halo carbonyl | –oyl halide |
| Acid amide | −CONH2 | Carbamoyl or Amido | Amide |
| Cyanide | −C ≡ N | Cyano | Nitrile |
| Iso cyanide |
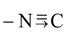 |
Iso cyano |
Iso nitrile or carbyl
amine |
| Aldehyde | −CHO |
Oxo, aldo (for aldehyde)
Or formyl (for CHO) |
–al |
| Ketone |
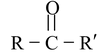 |
Oxo or keto | –one |
| Alcohol | −OH | Hydroxy | –ol |
| Thiol | –SH | Mercapto | Thiol |
| Amines | −NH2 | Amino | Amine |
| Ethers | −O− | Alkoxy | − |
| Alkene | = | – | ene |
| Alkyne | ≡ | – | yne |
| Nitro. | –NO2 | Nitro | – |
| Nitroso. | –N = O | Nitroso | – |
| Halogen | −X | Halo | − |
| Alkyl | –R | Alkyl | – |
- Introduction
- Nomenclature Of Organic Compound
- Naming Of Smaller Compounds
- Naming Of Bigger Compounds
- Naming Of Cyclic Compound
- Polycyclic Molecules
- Types Of Bond Cleavage
- Types Of Reagents
- Various Intermediates
- Inductive Effect
- Electromeric Effect
- Resonance Effect Of Mesomerism
- Hyperconjugation
- Application Of Inductive And Resonance Effect
- Strength Of Acids And Bases
- Relative Strength Of Acids And Bases
- Aromaticity
- Isomerism
- Structural Isomerism
- Stereo isomerism
- Conformation Of Butane
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4









