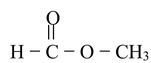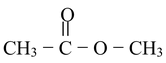Naming Of Smaller Compounds
GOC of Class 11
Naming Of Smaller Compounds
We shall take up the cases family wise. The first family is hydrocarbon. But it is a big family. For the sake of convenience it has been divided into three sub−groups.
(i) Saturated hydrocarbons having (C − C) bond.
(ii) Unsaturated hydrocarbons having (C = C) bond and
(iii) Unsaturated hydrocarbons having (C ≡C) bond.
In IUPAC rule, the last part in the name designates the family of the compound and the first part of the name indicates the number of carbon atoms in the parent chain of the compound.
(i) Hydrocarbons with only (C−C) bond
The first family is hydrocarbon having (C − C) bond and H atoms. This family has been given the name alkane. So the name of all saturated hydrocarbons end in −ane.
The name of the alkanes is given by writing the number of carbon atoms as prefix followed by suffix −ane.
|
Common name |
Molecular formula |
IUPAC name |
|
Methane |
CH4 |
Methane |
|
Ethane |
C2H6 |
Ethane |
|
Propane |
C3H8 |
Propane |
|
Butane |
C4H10 |
Butane |
|
Pentane |
C5H12 |
Pentane |
|
Hexane |
C6H14 |
Hexane |
|
Heptane |
C7H16 |
Heptane |
|
Octane |
C8H18 |
Octane |
|
Nonane |
C9H20 |
Nonane |
|
Decane |
C10H22 |
Decane |
Naming of alkyl radicals
When one hydrogen atom is removed from an alkane, the radical or group formed is called alkyl radical or group. The IUPAC name is given to such radicals by replacing −ane of alkane by −yl. Thus
−CH3 is called methyl (Me)
−C2H5 is called ethyl (Et)
CH3 − CH2 − CH2 is called n−propyl (n−Pr)
(CH3)2−C is called tertiary butyl or t−butyl (t–But)
(ii) Hydrocarbons with only (C = C) bond
The suffix of this family is −ene.
|
Common name |
Molecular formula |
IUPAC name |
|
Ethylene |
C2H4 |
Ethene |
|
Propylene |
C3H6 |
Propene |
|
Butylene |
C4H8 |
Butene |
|
Pentylene |
C5H10 |
Pentene |
|
Hexene |
C6H12 |
Hexene |
|
Heptene |
C7H14 |
Heptene |
|
Octene |
C8H16 |
Octene |
(iii) Hydrocarbons with only (C ≡ C) bond
The suffix of this family is −yne.
|
Common name |
Molecular formula |
IUPAC name |
|
Acetylene |
C2H2 |
Ethyne |
|
Methyl acetylene |
C3H4 |
Propyne |
|
Dimethyl acetylene |
C4H6 |
Butyne |
|
Ethyl methyl acetylene |
C5H8 |
Pentyne |
|
Hexyne |
C6H10 |
Hexyne |
|
Heptyne |
C7H12 |
Heptyne |
|
Octyne |
C8H14 |
Octyne |
(iv) Alcohol
The suffix of this family is −ol. So the general name ALKANOL.
|
Common name |
Molecular formula |
IUPAC name |
|
Methyl alcohol |
CH3OH |
Methanol |
|
Ethyl alcohol |
CH3CH2OH |
Ethanol |
|
Propyl alcohol |
CH3CH2CH2OH |
Propanol |
|
Butyl alcohol |
CH3CH2CH2CH2OH |
Butanol |
|
Pentanol |
CH3CH2CH2CH2CH2OH |
Pentanol |
|
Hexanol |
CH3CH2CH2CH2CH2CH2OH |
Hexanol |
(v) Ether
This compound is treated as the alkoxy derivative of alkane, so the word ALKOXY is written before the name of parent hydrocarbon.
R−O − R’
alkoxy alkane (where R = Smaller alkyl group; R’ = Bigger parent alkyl group)
IUPAC name: ALKOXY ALKANE
|
Common name |
Molecular formula |
IUPAC name |
|
Dimethyl ether |
CH3 − O − CH3 |
Methoxy methane |
|
Ethyl methyl ether |
CH3 − O − C2H5 |
Methoxy ethane |
|
Diethyl ether |
C2H5 − O – C2H5 |
Ethoxy ethane |
(vi) Aldehyde
The suffix of family is −al.
|
Common name |
Molecular formula |
IUPAC name |
|
Formaldehyde |
HCHO |
Methanal |
|
Acetaldehyde |
CH3CHO |
Ethanal |
|
Propionaldehyde |
CH3CH2CHO |
Propanal |
|
Butyraldehyde |
CH3CH2CH2CHO |
Butanal |
|
Pentanal |
CH3CH2CH2CH2CHO |
Pentanal |
|
Hexanal |
CH3CH2CH2CH2CH2CHO |
Hexanal |
(vii) Ketone
The suffix of family is −one.
|
Common name |
Molecular formula |
IUPAC name |
|
Acetone |
CH3COCH3 |
Propanone |
|
Ethyl methyl ketone |
CH3COC2H5 |
Butanone |
|
Diethyl ketone |
C2H5COC2H5 |
Pentanone |
|
Hexanone |
C2H5COC3H7 |
Hexanone |
|
Heptanone |
C3H7COC3H7 |
Heptanone |
(viii) Acid
The suffix of family is −oic acid.
|
Common name |
Molecular formula |
IUPAC name |
|
Formic acid |
HCOOH |
Methanoic acid |
|
Acetic acid |
CH3COOH |
Ethanoic acid |
|
Propionic acid |
CH3CH2COOH |
Propanoic acid |
|
Butanoic acid |
CH3CH2CH2COOH |
Butanoic acid |
|
Pentanoic acid |
CH3CH2CH2CH2COOH |
Pentanoic acid |
(ix) Amines
There are three types of amines−primary, secondary and tertiary
Primary amines are named by putting the word Amino before alkane.
The title of primary amine is Aminoalkane.
Secondary amines are named by putting the word Alkylamino before alkane.
The title of secondary amine is Alkylaminoalkane.
Tertiary amines are named by putting the word Dialkylamino before alkane.
The title of tertiary amine is Dialkylaminoalkane.
|
Common name |
Molecular formula |
IUPAC name |
|
Methyl amine |
CH3NH2 |
Amino methane |
|
Ethyl amine |
CH3CH2NH2 |
Amino ethane |
|
Dimethyl amine |
CH3 − NH − CH3 |
N−Methyl amino methane |
|
Trimethyl amine |
(CH3)2N − CH3 |
N,N−Dimethyl amino methane |
(x) Derivatives of Acid
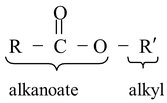
(a) Ester: The IUPAC name of ester is ALKYL ALKANOATE.
|
Common name |
Molecular formula |
IUPAC name |
|
Methyl formate |
|
Methyl methanoate |
|
Methyl acetate |
|
Methyl ethanoate |
(b) Amide: The IUPAC name is ALKANAMIDE.
Examples:
|
Common name |
Molecular formula |
IUPAC name |
|
Formamide |
HCONH2 |
Methanamide |
|
Acetamide |
CH3CONH2 |
Ethanamide |
|
Propionamide |
CH3CH2CONH2 |
Propanamide |
(c) Acid chloride: The IUPAC name is ALKANOYL CHLORIDE.
Examples:
|
Common name |
Molecular formula |
IUPAC name |
|
Formyl chloride |
HCOCl |
Methanoyl chloride |
|
Acetyl chloride |
CH3COCl |
Ethanoyl chloride |
|
Propionyl chloride |
CH3CH2COCl |
Propanoyl chloride |
(d) Acid Anhydride: The IUPAC name is ALKANOIC ANHYDRIDE.
To name a mixed anhydride alphabetical order is maintained.
|
Common name |
Molecular formula |
IUPAC name |
|
Acetic anhydride |
CH3−CO−O−CO−CH3 |
Ethanoic anhydride |
|
Propionic anhydride |
C2H5−CO−O−CO−C2H5 |
Propanoic anhydride |
|
Acetic propionic anhydride |
CH3−CO−O−CO−C2H5 |
Ethanoic propanoic anhydride |
- Introduction
- Nomenclature Of Organic Compound
- Naming Of Smaller Compounds
- Naming Of Bigger Compounds
- Naming Of Cyclic Compound
- Polycyclic Molecules
- Types Of Bond Cleavage
- Types Of Reagents
- Various Intermediates
- Inductive Effect
- Electromeric Effect
- Resonance Effect Of Mesomerism
- Hyperconjugation
- Application Of Inductive And Resonance Effect
- Strength Of Acids And Bases
- Relative Strength Of Acids And Bases
- Aromaticity
- Isomerism
- Structural Isomerism
- Stereo isomerism
- Conformation Of Butane
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4