Types Of Bond Cleavage
GOC of Class 11
Types Of Bond Cleavage
A covalent bond between two atoms can be broken in two ways−
-
Homolytic cleavage R − X
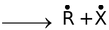
-
Heterolytic cleavage R − X
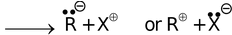
In the first cleavage, each atom separates with one electron leading to the formation of highly reactive intermediates called free radicals. In the second type of fission, one atom may hold on both the electrons leaving none for the other, resulting in a negative and a positive ion (or ion pair).
Homolytic cleavage occurs generally in the gas phase or in solution in non−polar solvents and is catalysed by ultra violet light or high temperature or by radical initiators like peroxides. Heterolytic cleavage occurs in polar solvents because of the ease of separation of charge and stabilization of the resultant ion pairs through solvation. In such heterolytic fission, the charged species formed are either carbon bearing positive charge called carbocation or carbon bearing negative charge called carbanion.
- Introduction
- Nomenclature Of Organic Compound
- Naming Of Smaller Compounds
- Naming Of Bigger Compounds
- Naming Of Cyclic Compound
- Polycyclic Molecules
- Types Of Bond Cleavage
- Types Of Reagents
- Various Intermediates
- Inductive Effect
- Electromeric Effect
- Resonance Effect Of Mesomerism
- Hyperconjugation
- Application Of Inductive And Resonance Effect
- Strength Of Acids And Bases
- Relative Strength Of Acids And Bases
- Aromaticity
- Isomerism
- Structural Isomerism
- Stereo isomerism
- Conformation Of Butane
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4









