Stereo isomerism
GOC of Class 11
In this isomerism the molecular formula as well as the structural formula are same, but the difference lies in the orientation of the atoms or group of atoms in space. This is divided into three subcategories.
(i) Conformational isomerism (ii) Optical isomerism (iii) Geometrical isomerism
Conformational Isomerism
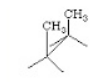
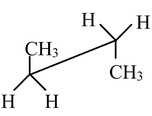
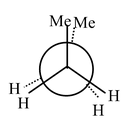
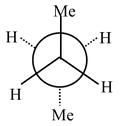
Carbon−carbon single bonds (σ bonds) are symmetrical around the axis and can be rotated freely in open chain molecules. Due to this free rotation of carbon−carbon single bonds, indefinite number of orientations in space are possible. These indefinite possibilities result in the formation of indefinite number of space isomers. These are called conformational isomers or rotomers which arises due to free rotation about a σ bond. Out of these indefinite arrangements only two extreme cases (staggered and eclipsed) are important. These can be represented on paper either according to Sawhorse representation or according to Newman projection. Conformational isomers of ethane and n−butane are represented below.
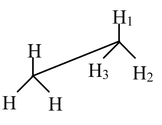
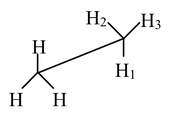
Sawhorse Representation of Ethane
|
Eclipsed |
Staggered |
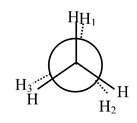
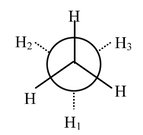
Newman Representation of Ethane
|
Eclipsed |
Staggered |
Sawhorse Representation of n−butane
|
Eclipsed |
Staggered |
Newman Representation of n−butane
|
Eclipsed |
Staggered |
In eclipsed conformer, the various groups will be at the minimum possible distance from each other whereas in staggered conformer the various groups will be at maximum distance from each other. The steric repulsions (due to overcrowding) will be maximum in eclipsed and minimum in staggered due to which the energy of the staggered conformer is lesser than that of eclipsed conformer. The staggered conformer is most stable whereas the eclipsed conformer is least stable. But the energy difference between the conformational isomers is very less which can be acquired by the molecular collisions. So the various conformational isomers keep on changing into one another and it is not possible to separate these conformational isomers. These conformational isomers are not counted while finding the total number of isomers for a given molecular formula but are useful whenever the stereochemical aspect of a chemical reaction is to be dealt.
- Introduction
- Nomenclature Of Organic Compound
- Naming Of Smaller Compounds
- Naming Of Bigger Compounds
- Naming Of Cyclic Compound
- Polycyclic Molecules
- Types Of Bond Cleavage
- Types Of Reagents
- Various Intermediates
- Inductive Effect
- Electromeric Effect
- Resonance Effect Of Mesomerism
- Hyperconjugation
- Application Of Inductive And Resonance Effect
- Strength Of Acids And Bases
- Relative Strength Of Acids And Bases
- Aromaticity
- Isomerism
- Structural Isomerism
- Stereo isomerism
- Conformation Of Butane
- Exercise 1
- Exercise 2
- Exercise 3
- Exercise 4