
Hyperconjugation
GOC of Class 11
As we have seen, the general inductive effect of alkyl groups is R3C > R2CH > RCH2 > CH3. This inductive order has been used satisfactorily to explain many phenomenon like the dipole moments in the gas phase of PhCH3, PhC2H5, PhCH(CH3)2 and PhC(CH3)3 are 0.37, 0.58, 0.65 and 0.70 D respectively. However, in some reactions, the inductive order is reversed. For example, the rate of reaction of p-substituted benzyl bromide with pyridine in acetone (follows SN2 pathway) is just opposite than that expected from the electron release by the inductive effect. That is, the methyl substituted compound reacted fastest and the t-butyl substituted compound reacted slowest. The rate order for the R groups was
Me > Et > iso−Pr > t-Bu.
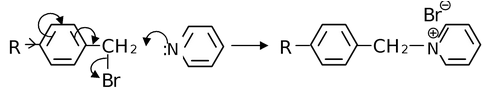
Thus, the order of electron release is exactly the reverse of inductive effect of alkyl groups. Therefore, alkyl groups must also possess some other mechanism of electron release in which the order is Me > Et > isoPr > t- Bu.
This came to be called as Hyperconjugation effect or Baker−Nathan effect (after its discoverer).
Thus, when the C ⎯ H bond is attached to an unsaturated carbon atom, the σ−electrons of the
C−H bond becomes less localized by entering into partial conjugation with the attached unsaturated system i.e. σ, π- conjugation.
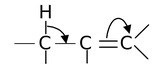
This type of conjugation between the σ−electrons of single bond and π−electrons of multiple bond is known as Hyperconjugation. It is a permanent effect which stabilizes the molecule.
When CH3 group is attached to an unsaturated atom or one with an unshared orbital, the canonical forms are drawn as
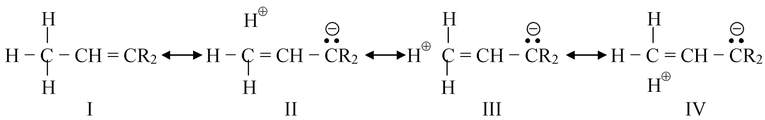
In such canonical forms there is no bond at all between the carbon and hydrogen but the hydrogen is not free as H+ because it will then contradict the condition of resonance, in which the relative position of the atoms in the molecule should not change. Thus, hyperconjugable hydrogens are not acidic in nature.
The effect of II, III and IV on the actual molecule is that the electrons in the C − H bond are closer to the carbon than they would be if II, III and IV did not contribute at all. For the other alkyl groups sequence –C2H5, –CH(CH3)2, –C(CH3)3, the hyperconjugation is further diminished because the number of C ⎯ H bonds decreases. Hence with respect to this effect, methyl is the strongest electron donor and t-butyl the weakest.
The significance of this effect is to explain certain facts like
(a) o,p-directing influence of CH3 group: In toluene, as CH3 is linked to an sp2 hybridized carbon, it does show hyperconjugation.
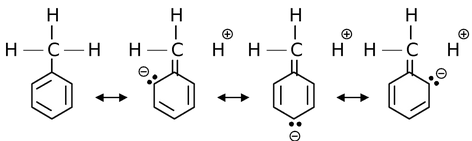
As it is evident from the hyperconjugable structures that the electron density will be high at ortho and para positions, so electrophile attack is favoured at these two positions in preference to meta attack. Thus, the directive influence of CH3 group is ortho & para.
(b) Relative stability of alkenes: The stability of alkenes can be decided by looking at the structure of alkenes. The alkene which has more hyperconjugable hydrogens will be more stable than the other with lesser hyperconjugable hydrogens. For example,

In 1-butene, the hyperconjugable structures possible are only 2 while with 2−butene, it will be 6. Thus, 2-butene is more stable than 1-butene.
In a reaction leading to the formation of both these alkenes, the 2-butene will be the major product while 1-butene will be the minor product. This is also decided by Saytzeff’s rule which is an outcome of the hyperconjugation only. Saytzeff rule states that the more substituted alkene will be the major product in a reaction which can give two or more alkenes. Thus, 2−butene being disubstituted would be the major product while 1−butene would be the minor product as it is monosubstituted.
(c) m-directing influence of –CCl3 group:
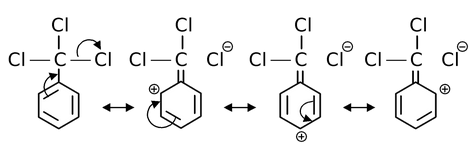
From these structures it is evident that the electron density being greater at meta position than at ortho and para, the electrophilic attack will be favoured at meta position. Thus, the directive influence of –CCl3 group is meta.
