Allotropic Forms Of Carbon
Inorganic Compound of Class 12
Allotropic Forms Of Carbon
The property due to which an element exists in two or more forms which differ in their physical and some of the chemical properties as known as allotropy and the various forms are called allotropes or allotropic modifications. This phenomenon is due to the difference either in the number of atoms in the molecules (as in the case of oxygen (O2) and ozone (O3)) or arrangement of atoms in the molecule (as in the case of various forms of carbon).
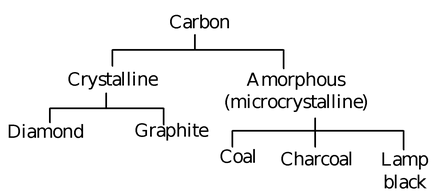
Carbon exists in two allotropic forms
(i) Crystalline (ii) amorphous
The crystalline forms are diamond and graphite while the amorphous forms are coal, charcoal, lamp black, etc.
Diamond
(i) It is the purest form of carbon.
(ii) It is found naturally as well as obtained artificially.
(iii) It is the hardest natural substance known.
(iv) It is transparent and has a specific gravity 3.52.
(v) It is a bad conductor of heat and electricity. It is transparent to X−rays and glows in ultraviolet rays with bluish green colour.
(vi) Its refractive index is high (2.45) and when properly cut, it produces maximum total internal reflection which is responsible for its brilliance.
(vii) It is chemically inert. It is not attacked by acids, alkalies and slats. It burns in air on heating at 900°C to form CO2. It reacts with fluorine at 700°C to form CF4.
C + O2 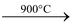 CO2
CO2
(Diamond)
C + 2F2 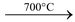 CF4
CF4
|
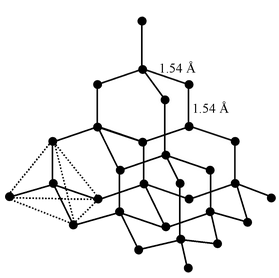
The important properties of diamond are related to its structure. In diamond, each carbon atom is in sp3 hybridized state and linked to four other carbon atoms tetrahedrally by covalent bonds. This gives a giant three dimensional polymeric structure in which C−C distance is 1.54 Å and bond angle is 109.5°. |
Structure of Diamond |
As the atoms are held firmly by strong covalent bonds, diamond is the hardest substance, possesses very high melting point (3600°C) and chemically inert. Since there is no mobile electron present, diamond is non−conductor of electricity.
Diamond is used as a gem stone on account of reflection and refraction of light. Impure diamonds (black) are used in knives for cutting glass and rock borers.
Graphite
(i) It is a soft, greasy, dark grayish coloured crystalline solid.
(ii) Its density is 2.5 g ml−1.
(iii) It is good conductor of electricity and its conductivity increases with temperature.
(iv) It leaves a black mark on paper and is called black lead or plumbago.
(v) It is chemically more active than diamond. It ignites in air or oxygen at 700°C to form CO2. It is not attacked by alkalies and dilute acids. However, when treated with concentrated HNO3 or concentrated H2SO4, it is oxidized to insoluble yellowish green substance known as graphitic acid, C11H4O5. With alkaline potassium permanganate, it is oxidized to mellitic acid [C6(COOH)6] and oxalic acid. It forms CO2 with chromic acid.
Uses
(i) For lining and making electrodes of electric furnaces.
(ii) In making refractory crucibles.
(iii) In making lead pencils.
(iv) As a moderator in nuclear reactor.
(v) As a lubricant in machinery.