
Water
Inorganic Compound of Class 12
Structure
|
Water molecule has V−shaped structure with sp3 hybridization of oxygen atom. Bond angle is 105° in place of 109°28’ (normal bond angle of tetrahedron) because of the presence of two lone pairs. |
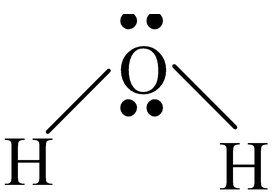 |
Properties
Water is a very stable liquid with a very high dielectric constant. It is therefore known as universal solvent.
Hard and Soft water
ater which lathers easily on shaking with soap solution is called soft water. Such a water does not contain dissolved calcium and magnesium salts in it.
n the other hand hard water does not lather easily with soap solution. This is due to the presence of certain salts of calcium, magnesium and other heavy metals dissolved in it.
sample of hard water when treated with soap (salts of higher fatty acids) do not produce lather but if forms a white precipitate or scum as follows
2C17H35COONa + CaC22 → (C17H35COO)2Ca↓ + 2NaCl
Sodium sterate (Hardness) Calcium stearate
(soap) (insoluble)
Water resource: Hardness of water may be of two types
(i) Temporary hardness: This is caused by the presence of dissolved bicarbonates of calcium, magnesium and other heavy metals and carbonates of iron.
Temporary hardness is mostly destroyed by mere boiling of water, when bicarbonates are decomposed yielding insoluble carbonates or hydroxides.
Ca(HCO3)2 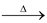 CaCO3↓ + H2O + CO2↑
CaCO3↓ + H2O + CO2↑
Calcium Calcium
bicarbonate carbonate
(soluble) (insoluble)
(ii) Permanent Hardness: This is due to the presence of chlorides and sulphates of calcium, magnesium, iron and other heavy metals. Unlike temporary hardness, permanent hardness is not destroyed on boiling.
But it may be destroyed by using lime−soda process as follows
