
Oxides Of Carbon
Inorganic Compound of Class 12
Oxides Of Carbon
Two oxides of carbon, carbon monoxide and carbon dioxide are important and described below.
(i) Carbon Monoxide, CO
It is found in small amounts in volcanic gases, chimney gases, exhaust gases of internal combustion engines and coal gas.
Preparation
The following methods can be applied for the preparation of carbon monoxide.
(a) By heating oxalic acid with concentrated sulphuric acid: A mixture of CO and CO2 is obtained. Sulphuric acid acts as a dehydrating agent. CO2 is removed by passing the gaseous mixture through caustic soda or caustic potash solution.
(COOH)2 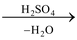 CO + CO2
CO + CO2
Similarly by heating formic acid with concentrated H2SO4, only CO is obtained.
HCOOH 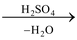 CO
CO
(b) By the reduction of oxides of heavy metals with carbon: On heating oxides of heavy metals with carbon, CO is formed.
Fe2O3 + 3C  2Fe + 3CO
2Fe + 3CO
ZnO + C  Zn + CO
Zn + CO
(c) By reduction of carbon dioxide: CO2 can be reduced with carbon or zinc at high temperatures. CO2 when passed over red hot zinc, a mixture of CO and CO2 is obtained.
Zn + CO2 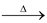 ZnO + CO
ZnO + CO
(d) By heating carbonates of calcium, barium or magnesium with zinc: CO is obtained.
MgCO3 + Zn  MgO + ZnO + CO
MgO + ZnO + CO
(e) By heating potassium ferrocyanide with conc. H2SO4: When potassium ferrocyanide in powdered state is heated with concentrated H2SO4, CO is evolved. Dilute H2SO4 should never be used because it shall evolve highly poisonous gas, HCN.
K4Fe(CN)6 + 6H2SO4 + 6H2O  2K2SO4 + FeSO4 + 6CO + 3(NH4)2SO4
2K2SO4 + FeSO4 + 6CO + 3(NH4)2SO4
Properties
(i) It is a colourless and odourless gas.
(ii) It is slightly soluble in water.
(iii) It is a combustible gas but does not support combustion.
(iv) Its density is nearly equal to the density of air.
(v) It is highly poisonous in nature. One part in 100 parts of air causes death in few minutes. The poisonous nature of CO is due to the fact that it combines with haemoglobin (a red colouring matter of blood which is absorber of oxygen) to form carboxy−haemoglobin which is not capable to absorb oxygen and as a result of this, suffocation takes place.
(vi) It is not decomposed by heat.
(vii) It is neutral to litmus.
(viii) It burns with blue flame to form CO2. This is an exothermic process.
2CO + O2  2CO2 + heat
2CO2 + heat
(ix) It is a good reducing agent as it takes up oxygen and converted into CO2.
(x) The bonding in carbon monoxide is represented as
: C : : : O : or : C 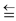 O
O
Thus, all the four valencies of carbon are not satisfied. It behaves as unsaturated compound and forms addition products with a number of substances.
CO + 2H2 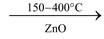 CH3OH
CH3OH
Methyl alcohol
CO + Cl2  COCl2
COCl2
Phosgene
(xi) It combines with metals like Cr, Ni, Fe, etc. The compounds thus formed are called carbonyls.
Ni + 4CO  Ni(CO)4
Ni(CO)4
Fe + 5CO 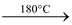 Fe(CO)5
Fe(CO)5
These reactions are used in the extraction and purification of metals.
Uses
(i) It is used as a fuel in the form of water gas (CO + H2) and producer gas (CO + N2).
(ii) CO is used in the manufacture of methanol, synthetic petrol, formic acid and phosgene gas (highly poisonous gas).
(iii) It is used as a reducing agent in the extraction of iron.
(iv) It is used in the extraction of nickel (Mond’s process).
(ii) Carbon dioxide, CO2
Occurrence: It is present in atmosphere to the extent of 0.03−0.05 percent. It comes to the atmosphere from animal breathing, decay of vegetable matter, burning of carbon and carbonous matter etc. It is also utilized by plants in photosynthesis. Thus, a carbon dioxide cycle is operating in nature and the proportion of CO2 in the atmosphere remains about the same. It is found in combined state in the form of carbonates.
Preparation
The following method are used for its preparation.
(a) By the complete combustion of carbon: Carbon is burnt in free supply of air.
C + O2  CO2
CO2
If CO is formed, it also burns with pale blue flame forming carbon dioxide.
2C + O2  2CO
2CO
2CO + O2  2CO2
2CO2
(b) By the action of dil. mineral acids on carbonates and bicarbonates: Mineral acids react with carbonates and bicarbonates and evolve carbon dioxide.
CaCO3 + 2HCl  CaCl2 + H2O + CO2
CaCl2 + H2O + CO2
Na2CO3 + 2HCl  2NaCl + H2O + CO2
2NaCl + H2O + CO2
NaHCO3 + HCl  NaCl + H2O + CO2
NaCl + H2O + CO2
(c) By heating carbonates and bicarbonates: the carbonates of less electropositive metals on heating decompose evolving carbon dioxide.
ZnCO3  ZnO + CO2
ZnO + CO2
CuCO3  CuO + CO2
CuO + CO2
Bicarbonates of all the metals decompose on heating with evolution of CO2.
2NaHCO3  Na2CO3 + H2O + CO2
Na2CO3 + H2O + CO2
Ca(HCO3)2  CaCO3 + H2O + CO2
CaCO3 + H2O + CO2
Properties
(i) It is a colourless, odourless and tasteless gas.
(ii) It is slightly soluble in water under ordinary pressure but at high pressures, the solubility is high.
(iii) It is heavier than air.
(iv) It is easily liquefied under pressure into a colourless mobile liquid. If CO2 under pressure is allowed to escape through a nozzle, a white solid, i.e. dry ice is obtained. Solid CO2 is a soft, white, snow like substance. It sublimes and leaves no residue. Solid CO2 is used as a refrigerant under the commercial name drikold. It is used in the transport of perishable food materials. It provides cold as well as the inert atmosphere which helps in killing the undesirable bacteria.
(v) It is neither combustible nor a supporter of combustion. However, burning magnesium, sodium or potassium continues burning in the gas.
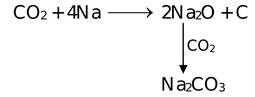
CO2 + 2Mg  2MgO + C
2MgO + C
(vi) CO2 acts as an oxidizing agent. When heated with Zn, iron or carbon, these are oxidized.
Zn + CO2  ZnO + CO
ZnO + CO
CO2 + C  2CO
2CO
(vii) CO2 is an acidic oxide. It dissolve in water forming unstable carbonic acid.
H2O + CO2 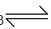 H2CO3
H2CO3
It combines with alkalies to form two series of salts, carbonates and bicarbonates.
2NaOH + CO2  Na2CO3 + H2O
Na2CO3 + H2O
With excess of CO2, carbonate is converted into bicarbonate.
Na2CO3 + H2O + CO2  2NaHCO3
2NaHCO3
(viii) CO2 reacts with basic oxides like Na2O, K2O etc. to form corresponding carbonates.
K2O + CO2  K2CO3
K2CO3
Na2O + CO2  Na2CO3
Na2CO3
(ix) CO2 is converted by plants in the presence of sunlight and chlorophyll into glucose and higher carbohydrates. This process is known as photosynthesis.
6CO2 + 6H2O  C6H12O6 + 6O2
C6H12O6 + 6O2
(x) Carbon forms some less stable oxides, e.g., C3O2, C5O2 and Cl12O9. Graphite oxides are C2O and C2O3 which are still less stable. Carbon suboxide is formed by dehydration of malonic acid.
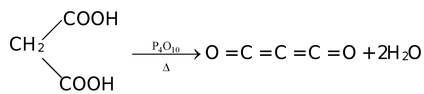
Uses
(i) It is used in the manufacture of aerated water.
(ii) Solid CO2 (dry ice) is used in refrigeration. It is superior refrigerant than common ice because it can produce very low temperatures and does not form liquid on melting.
(iii) Carbogen is a mixture of O2 and CO2 (5−10%). It is used for artificial respiration in the case of pneumonia patients and victims of CO poisoning.
(iv) It is used in the manufacture of white lead and sodium carbonate (Solvay process).
(v) CO2 is used as fire extinguisher. The use of common fire extinguisher is based on the production of CO2.
(vi) CO2 is used by plants in the form of food.
