
Plaster Of Paris
Inorganic Compound of Class 12
Plaster Of Paris
Plaster of Paris is calcium sulphate hemihydrate CaSO4.½H2O
Preparation
It is prepared by heating gypsum to 393 to 403 K.
2(CaSO4.2H2O) 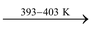 2CaSO4.H2O + 3H2O
2CaSO4.H2O + 3H2O
GypsumPlaster of Paris
The following conditions are necessary
(i) The temperature should not be allowed to rise above 390 K because above this temperature the whole of water of crystallization is lost. The resulting anhydrous CaSO4 is called dead burnt plaster because it loses the properties of setting with water.
(ii) The gypsum should not be allowed to come in contact with carbon containing fuel otherwise some of it will be reduced to calcium sulphite.
Properties
(i) It is a white powder.
(ii) On mixing with three times of its weight of water, it forms a plastic mass which sets into a hard solid within 5 to 15 minutes. It is due to this reason that it is called plaster. The setting of Plaster of Paris is believed to be due to rehydration and its reconversion into gypsum.
2CaSO4.H2O + 3H2O  2(CaSO4.2H2O)
2(CaSO4.2H2O)
Plaster of Paris Gypsum
During the process of setting, slight expansion (1%) in volume occurs. As a result, it can take the shape and impression of the mould in which it is put.
Uses
(i) Plaster of Paris is used for producing moulds for pottery and ceramics.
(ii) It is used for making statues, models and other decorative materials.
(iii) It is used in surgical bandages used for plastering broken or fractured bones of the body and for preparing black board chalks.
