
Sodium Bicarbonate (Baking Soda) NaHCO3
Inorganic Compound of Class 12
Sodium Bicarbonate
It is obtained as the intermediate product in the solvay ammonia soda process. Normal carbonate can be changed to bicarbonate by passing carbon dioxide through its saturated solution.
Na2CO3 + CO2 + H2O  2NaHCO3
2NaHCO3
Sparingly soluble
Properties
It is a white crystalline solid, sparingly soluble in water. The solution is alkaline in nature due to hydrolysis. The solution is weakly basic.
NaHCO3 + H2O 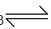 NaOH + H2CO3
NaOH + H2CO3
The solution gives yellow colour with methyl orange but no colour with phenolphthalein.
On heating, it loses carbon dioxide and water forming sodium carbonate.
2NaHCO3 Na2CO3 + H2O + CO2
Na2CO3 + H2O + CO2
The metal salt which forms basic carbonate with sodium carbonate, gives normal carbonate with sodium bicarbonate.
ZnSO4 + 2NaHCO3 ZnCO3 + Na2SO4 + H2O + CO2
ZnCO3 + Na2SO4 + H2O + CO2
Uses
(i) It is used as a medicine (sodabicarb) to neutralize the acidity in the stomach.
(ii) It is largely used for making baking powder. Baking powder is a mixture of potassium hydrogen tartrate and sodium bicarbonate.
(iii) It is used in making effervescent drinks.
(iv) It is used in fire extinguishers.
(v) It is used for production of carbon dioxide.
