
Borax
Inorganic Compound of Class 12
Preparation
It is prepared from naturally occuring calcium Borate. The mineral is boiled with concentrated solution of Na2CO3. The solution obtained in filteration is concentrated when crystals of Borax are deposited.
Ca2B6O11 + 2Na2CO3  3CaCO3↓ + Na2B4O7 + 2NaBO2
3CaCO3↓ + Na2B4O7 + 2NaBO2
CaCO3 is filtered off. White needles of Borax separate out when hot solution is cooled.
Na2B4O7 + NaBO2 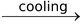 Na2B4O7↓ + NaBO2
Na2B4O7↓ + NaBO2
Hotseparates
as white needle
On passing CO2 gas to mother liquor containing NaBO2 more Borax gets deposited.
4NaBO2 + CO2  Na2B4O7↓ + Na2CO3
Na2B4O7↓ + Na2CO3
Borax
Properties
(i) White needle having greater solubility in water. (Particularly in hot water)
(ii) Its aqueous solution is alkaline because of formation of strong base NaOH and weak acid H3BO4. Therefore it can be titrated against strong mineral acid useing methyl orange as indicator.
Na2B4O7 + 7H2O  2NaOH + 4H3BO3
2NaOH + 4H3BO3
strong Boric acid (weak acid)
base
BORAX BEAD TEST (only given by coloured salts)
Na2B4O7.10H2O 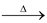 Na2B4O7 + 10H2O
Na2B4O7 + 10H2O
Na2B4O7 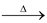 NaBO2 + B2O3
NaBO2 + B2O3
sodium Boron trioxide
meta Borate Borax Bead
(colourless, glassy)
This glassy bead reacts with a number of oxides giving characteristic coloured bead so this test is called Borax Bead test.
Cr2O3 + B2O3  Cr(BO2)3
Cr(BO2)3
Chromium meta borate
(green bead)
NiO + B2O3  Ni(BO2)2
Ni(BO2)2
(yellowish brown bead)
Nickel meta Borate
CuO + B2O3  Cu(BO2)2
Cu(BO2)2
(Blue bead)
(Cu−Meta Borate)
If this blue bead is heated in a reduction flame it turns red due to formation of metallic copper.
CoO + B2O3  Co(BO2)2
Co(BO2)2
(Blue)
Mn(BO2)4 is pink and Mn(BO2)2 is colourless in reduction flame.
INORGANIC GRAPHITE
Boron nitride, BN has a graphite−like layer structure of alternate B and N and hence it is known as inorganic graphite. It is prepared by the following reaction
Na2B4O7 + 2NH4Cl  2BN + B2O3 + 2NaCl + 4H2O
2BN + B2O3 + 2NaCl + 4H2O
INORGANIC BENZENE
Borazole, B3N3H6 is an inorganic compound which possesses a cyclic ring structure similar to benzene with alternate single and double bonds. That is why it is called inorganic benzene.
3BCl3 + 3NH4Cl  B3N3H3Cl3
B3N3H3Cl3 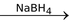 B3N3H6
B3N3H6
Borazole
