
Calcium Carbonate Limestone Or Marble
Inorganic Compound of Class 12
Preparation
(i) From slaked lime: It is prepared by passing equimolar amount of carbon dioxide through slaked lime.
Ca(OH)2 + CO2  CaCO3↓ + H2O
CaCO3↓ + H2O
(ii) From calcium chloride: It is prepared by adding an aqueous solution of sodium carbonate to calcium chloride.
Na2CO3 + CaCl2  CaCO3↓ + 2NaCl
CaCO3↓ + 2NaCl
Properties
(i) It is a white fluffy powder almost insoluble in water.
(ii) When heated to 1270 K, it decomposes to form quick lime.
CaCO3 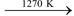 CaO + CO2
CaO + CO2
(iii) It reacts with dilute acids evolving CO2 gas.
CaCO3 + 2HCl  CaCl2 + H2O + CO2
CaCl2 + H2O + CO2
CaCO3 + H2SO4  CaSO4 + H2O + CO2
CaSO4 + H2O + CO2
Uses
(i) Calcium carbonate is used as a building material in form of marble.
(ii) It is also used as a raw material for the manufacture of sodium carbonate by Solvay−ammonia process.
(iii) In pure and finely powdered form, it is used in tooth paste and cosmetics.
(iv) It is used in agriculture for neutralizing the acidity of soil.
