
Calcium Oxide CaO
Inorganic Compound of Class 12
It is also called quick lime.
Preparation
It is prepared by heating limestone in a rotatory kiln at 1070−1270 K.
CaCO3(s) 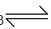 CaO(s) + CO2(g);ΔH = +179.9 kJ
CaO(s) + CO2(g);ΔH = +179.9 kJ
The necessary conditions for obtaining a good yield of quick lime are:
(i) Since the reaction is reversible, carbon dioxide should be removed as soon as it is formed in order to shift the equilibrium in the forward direction in accordance with
Le Chatelier’s Principle.
(ii) The temperature should not be allowed to rise above 1270 K otherwise silica present as impurity in lime will combine with calcium oxide to form infusible calcium silicate.
CaO + SiO2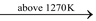 CaSiO3
CaSiO3
(calcium silicate)
Properties
(i) It is a white amorphous solid with melting point of 2870 K.
(ii) When exposed to atmosphere, it absorbs moisture and carbon dioxide forming slaked lime and calcium carbonate respectively.
CaO + H2O(moisture)  Ca(OH)2
Ca(OH)2
(slaked lime)
CaO + CO2 CaCO3 (calcium carbonate)
CaCO3 (calcium carbonate)
(iii) On adding water, it produces a hissing sound and a large amount of heat is evolved which converts water into steam. This process is called slaking of lime and the fine powder thus obtained is called slaked lime.
CaO + H2O  Ca(OH)2;ΔH = −63 kJ
Ca(OH)2;ΔH = −63 kJ
(iv) Action of acids and acidic oxides: It is a basic oxide and hence combines with acids and acidic oxides forming salts.
CaO + 2HCl  CaCl2 + H2O
CaCl2 + H2O
CaO + SO2 CaSO3
CaSO3
(v) Reaction with coke: When treated with coke in an electric furnace at 2273−3273 K, it forms calcium carbide.
CaO + 3C 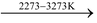 CaC2 + CO
CaC2 + CO
(vi) Reaction with ammonium salts: On heating with ammonium salts, it liberates ammonia gas.
CaO + 2NH4Cl  CaCl2 + 2NH3 + H2O
CaCl2 + 2NH3 + H2O
Uses
(i) It is used as a building material.
(ii) In the preparation of cement, glass, calcium carbide and sodium carbonate (from caustic soda).
(iii) It is used in the preparation of ammonia and soda−lime (CaO + NaOH).
(iv) It is used for drying alcohols and non−acidic gases.
(v) It is used as basic lining in furnaces.
