
Ferric Chloride
Inorganic Compound of Class 12
Ferric Chloride
Anhydrous ferric chloride is prepared by passing dry Cl2 over heated iron filing when the salt sublimes at 315°C in a receiver as iron−black crystals with a green iridescence.
2Fe + 3Cl2 → 2FeCl3
In solution it is obtained by dissolving Fe2O3 or Fe(OH)3 in HCl or by saturating a solution of FeCl2 with Cl2.
Fe2O3 + 6HCl → 2FeCl3 + 3H2OFe(OH)3 + 3HCl → FeCl3 + 3H2O
2FeCl2 + Cl2 → 2FeCl3
The solution on evaporation and cooling deposits yellow crystals of the hydrate, FeCl3.6H2O or Fe2Cl6.12H2O. Other hydrates that separate from the aqueous solution contain Fe2Cl6 with 7, 5 and 4 molecules of water.
Properties
(i) FeCl3 is not an ionic compound but is a covalent compound as is indicated by its volatility and solubility in non−polar solvents like ether and alcohol. However, it dissolves in H2O to give Fe3+ and Cl ions.
(ii) Anhydrous FeCl3 forms iron black crystals with a green iridescence. These crystals sublime at 283C and its vapour density at 450C corresponds to the formula Fe2Cl6. At high temperatures dissociation of Fe2Cl6 occurs into FeCl3 molecules so that its vapour density progressively decreases on heating and corresponds to the formula FeCl3 at 750C. At higher temperatures the vapour density further decreases, probably due to the dissociation of FeCl3 into FeCl2 and Cl2.
Fe2Cl6![]() 2FeCl3
2FeCl3![]() 2FeCl2 + Cl2
2FeCl2 + Cl2
(iii) Formation of double salts: It forms a number of double salts with other chlorides, e.g. FeCl3.2KCl.H2O, FeCl3.2NH4Cl.H2O, FeCl3.MgCl2.H2O etc.
(iv)Oxidising properties: It is an oxidizing agent and oxidizes various substances. In these reactions it itself is reduced to FeCl2. Thus it oxidizes SnCl2 to SnCl4SO2 to H2SO4, H2S to S, nascent hydrogen to HCl and liberates I2 from KI solution.
2FeCl3 + SnCl2 → SnCl4+ 2FeCl2
SO2+ 2FeCl3 + 2H2O → H2SO4 + 2HCl + 2FeCl2
H2S + 2FeCl3 → S + 2HCl + 2FeCl2
FeCl3+ H → FeCl2 + HCl2FeCl3 + 2KI → 2KCl + I2 + 2FeCl2
(v) Action of Na2S2O3FeCl3 reacts with sodium thiosulphate, Na2S2O3 to form sodium tetrathionate, Na2S4O6.
2FeCl3+ 2Na2S2O3 → 2NaCl + Na2S4O6 + 2FeCl2
(vi) It is hygroscopic and dissolves readily in H2O, the solution is strongly acidic due to hydrolysis.
FeCl3 + 3H2O ![]() Fe(OH)3 + 3HCl
Fe(OH)3 + 3HCl
(vii) Action of NH3 and NOCl: It absorbs NH3 and nitrosyl chloride, NOCl to form FeCl3.6NH3 and FeCl3.NOCl respectively.
(viii) Formation of various hydrates: FeCl3 solution deposits crystals of various hydrates at various temperatures: Fe2Cl6.12H2, Fe2Cl6.7H2O (52.5C), Fe2Cl6.5H2O (56C), Fe2Cl6.2H2O (73.50C).
(ix) Action of heat: On heating FeCl3.6H2O is changed into Fe2O3 and H2Oand HCl are liberated.
2[FeCl3".6H2O 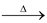 Fe2O3 + 9H2O + 6HCl
Fe2O3 + 9H2O + 6HCl
This reaction clearly shows that anhydrous FeCl3 cannot be prepared by simply heating the hydrated ferric chloride, FeCl3.6H2O.
Structure: Ferric chloride is a covalent compound and a dimerised molecule, Fe2Cl6 which has the structure shown below.
![]()
Structure of Fe2Cl6
Uses
(i) Ferric chloride solution coagulates blood and hence is used as a styptic, i.e. in stopping bleeding.
(ii) It is also used as an oxidizing agent in organic dye stuff industry.
(iii) It is also used as a mordant in dyeing.
DICHROMATE(![]() )
)
Two tetrahedral 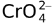 units are joined together.
units are joined together.
![]()
The structure of Rb2Cr2O7 indicates the following structure for ![]() .
.
![]()
