
Nitric Acid
Inorganic Compound of Class 12
Nitric Acid
In the laboratory it is prepared by heating conc. H2SO4 and an alkali nitrate in a glass retort.
Manufacture From Nitre(NaNO3):
Nitric acid is prepared in a large scale by heating sodium nitrate with concentrated sulphuric acid. The mixture is placed in a cast iron retort and subjected to distillation at a temperature of about 200°C.
The vapours of nitric acid from the pot are conducted to a trap and then passed through water−cooled silica pipes. The acid condensed in the pipes is sent back to the trap. The uncondensed vapours of nitric acid are then finally passed up a tower packed with quartz from the top of which a steam of water is trickling down. The vapours are dissolved in water and the dilute nitric acid thus produced collects at the bottom.
The residue left in the pot is known as nitre cakeand is used in bleaching and dyeing industries. It is also used for the preparation of hydrochloric acid.The acid so obtained is concentrated by distilling the dilute acid with concentrated sulphuric acids. Commerical nitric acid contains hydrochloric acids, sulphuric acid, iodine, iron salts and nitrogen pentoxide as impurities. A current of dry air passed through the liquid removes N2O5. Further purification is carried out by distilling the acid under reduced pressure.
Preparation of concentrated nitric acid:
The dilute HNO3can be concentrated to 68% by distillation, when constant boiling mixture is formed. This constant boiling mixture of HNO3has a specific gravity of 1.42 and is the commercial concentrated HNO3.More concentrated acid needed for certain uses such as nitration is produced by distillation with conc. H2SO4which holds back the water. The distillate is 98% HNO3. The cent percent HNO3is obtained by strongly cooling 98% acid when the pure acid is deposited as colourless crystals at −42°C. These crystals are separated and melted to 100% HNO3.
A recently developed method for the direct production of conc. HNO3involves the reaction between the liquid NO2and water (or dil. HNO3) in the presence of O2at 50 atm pressure and a temperature of 75°C. The product is 88% HNO3.
4NO2+ O2+ 2H2O 4HNO3
4HNO3
Preparation of fuming nitric acid:
It is made by distilling conc. HNO3 with a little starch. The starch reduces some acid to NO2which dissolves in the remaining acid to form fuming nitric acid. This acid is yellow in colour due to the presence in it of dissolved oxides of nitrogen highly corrosive liquid consisting of HNO3. Fuming nitric acid is a much more powerful oxidizing agent and nitrating agent than conc. HNO3
Physical Properties:
Pure HNO3 is a colourless liquid with a characteristic choking smell. It boils at 86°C and freezes at −42°C into a transparent crystalline mass. It is soluble in water in all proportions. In the moist air, it fumes as its vapours form a mist of minute droplets of aqueous HNO3.
Chemical Properties:
Oxidizing property: Nitric acid is a strong oxidizing agent because of the ease with which it decomposes to give nascent oxygen.
2HNO3 2NO2+ H2 O + O
2NO2+ H2 O + O
Since NO2 gas is produced and this gas remains dissolved, the colour of the acid become yellow or even reddish yellow in higher concentration. The oxidizing properties of the acid are indicated by its action on non−metals, metalloids, metals and on various compounds as shown below:
Action on non−metals: It oxidizes non−metals like sulphur, carbon, phosphorus and iodine to their corresponding oxy−acids. For example,
Sulphur is oxidized to sulphuric acid
S + 6HNO3 H2SO4+ 6NO2+ 2H2 O
H2SO4+ 6NO2+ 2H2 O
Carbon is oxidized to carbonic acid
C + 4HNO3 H2CO3 + 4NO2 + H2O
H2CO3 + 4NO2 + H2O
Iodine is oxidized to iodic acid (HIO3)
I2 + 10HNO3 2HIO3 + 10NO2 + 4H2O
2HIO3 + 10NO2 + 4H2O
Phosphorus is oxidized to ortho−phosphoric acid (H3PO4)
P + 5HNO3 H3PO4 + 5NO2 + H2O
H3PO4 + 5NO2 + H2O
Action on metalloids: Metalloids like arsenic and antimony are oxidized to the corresponding oxy−acids. e.g., arsenic is oxidized to ortho−arsenic acid (H3AsO4).
As + 5HNO3 H3AsO4 + 5NO2 + H2O
H3AsO4 + 5NO2 + H2O
Action on metals: Most of the metals, with the exception of noble metals like gold and platinum are attacked by nitric acid. Some like tin and antimony give oxides while the others form nitrates. During the reaction, a part of the acid is reduced to give products like NO2,NO,N2,N2O,NH2OH or NH3and the nature of products depends upon the concentration of the acid, temperature and the nature of the metal.
It is believed that the primary reaction in each case is the liberation of nascent hydrogen which then reduces the acid to different reaction products mentioned above.
M + HNO3 MNO3 + H
MNO3 + H
According to another view, the reduction occurs only in case of metals which are above hydrogen in the electrochemical series. Metals like Cu, Ag, Hg which cannot displace hydrogen are oxidized by nitric acid and the oxide formed, being more basic, reacts with more of nitric acid to form the nitrate. Examples are
Copper: With dilute nitric acid, it gives nitric oxide.
3Cu + 8HNO 3Cu(NO3)2 + 2NO + 4H2O
3Cu(NO3)2 + 2NO + 4H2O
With concentrated nitric acid, it forms nitrogen peroxide.
Cu + 4HNO3 Cu(NO3)2 + 2NO2 + 2H2O
Cu(NO3)2 + 2NO2 + 2H2O
Silver, mercury and lead give similar reactions.
Tin: With dilute nitric acid, it forms stannous nitrate and ammonium nitrate.
4Sn + 10HNO3 4Sn(NO3)O2 + 3H2O + NH4NO3
4Sn(NO3)O2 + 3H2O + NH4NO3
With concentrated nitric acid it gives meta−stannic acid and nitrogen peroxide.
Sn + 4HNO3 H2SnO3 + H2O + 4NO2
H2SnO3 + H2O + 4NO2
Meta stannic acid
Zinc: With very dilute nitric acid it gives ammonium nitrate.
4Zn + 10HNO3  4ZN(NO3)2 + 3H2O + NH4NO3
4ZN(NO3)2 + 3H2O + NH4NO3
With dilute acid, it produces N2O while concentrated acid gives NO2.
Magnesium and manganese are the only metals that liberate hydrogen from dilute acid.
2HNO3 + Mg  Mg(NO3)2 + H2
Mg(NO3)2 + H2
dilute
Gold and platinum dissolve in a mixture of 1 part HNO3and 3 parts HCl known as aqua regia. The dissolution occurs as a result of the action of nascent chlorine on the metals.
HNO3+ 3HCl  NOCl + 2H2O + 2Cl
NOCl + 2H2O + 2Cl
Action on compounds: A number of compounds are oxidized. For example it oxidizes:
Sulphur dioxide to the sulphuric acid
SO2 + 2HNO3 H2SO4 + 2NO2
H2SO4 + 2NO2
Sulphuretted hydrogen to sulphur
H2S + 2HNO3 2H2O + 2NO2 + S
2H2O + 2NO2 + S
Ferrous sulphate to ferric sulphate in the presence of sulphuric acid
6FeSO4 + 2HNO3 + 3H2SO4 3Fe2(SO4)3 + 4H2O + 2NO
3Fe2(SO4)3 + 4H2O + 2NO
The nitric oxide formed gives a dark brown ring (Ring test for nitrates).
Cane sugar to oxalic acid
C12H22O1118O 6(COOH)2 + 5H2O
6(COOH)2 + 5H2O
Nitrating property:
It reacts with organic compounds and forms their nitro compounds. The reaction takes place in the presence of stronger acids like conc. H2SO4.
In the presence of an acid which is stronger than nitric acid, NO2+ ions (nitronium ions) are produced.
HNO3 + 2H2SO4 NO2+H2SO4- + H3O+
NO2+H2SO4- + H3O+
When NO2+ ions replace the hydorgne ions in organic compounds, nitration is said to take place. Thus,
C6H6 + HNO3![]() C6 H5NO2 + H2O
C6 H5NO2 + H2O
benzene nitrobenzene
The yellow strain produced on wood or skin is due to this reaction.
Action on HCl:
A mixture of conc. HCl and conc. HNO3(3 : 1) is called aqua regia. It reacts considerably more vigorously than either of these acids taken separately.
HNO3 + 3HCl  2H2O + NOCl + 2Cl
2H2O + NOCl + 2Cl
Nitrosyl
chloride
Aqua regia dissolves even noble metals like gold and platinum.
Au + HNO3 + 3HCl  AuCl3 + NO + 2H2 O
AuCl3 + NO + 2H2 O
3Pt + 4HNO3 + 12HCl  3PtCl4 + 4NO + 8H2O
3PtCl4 + 4NO + 8H2O
The cause of greater activity of aqua regia is the formation of nascent chlorine at the moment of formation.
Acidic property:
Nitric acid is a strong acid. It ionizes in aqueous solution as
HNO3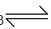 H+NO3-
H+NO3-
It decomposes metallic oxides, carbonates, bicarbonates, sulphides, sulphites, bisulphites and hydroxides forming metal nitrates. H2S liberated in reaction between HNO3and a sulphide further reacts with HNO3to give sulphur.
CuO + 2HNO3 Cu(NO3)O2 + H2O
Cu(NO3)O2 + H2O
Na2CO3 + HNO3 2NaNO3 + H2O + CO2
2NaNO3 + H2O + CO2
NaHCO3 + HNO3 NaNO3 + H2O + CO2
NaNO3 + H2O + CO2
Na2S + 2HNO3 2NaNO3 + H2S
2NaNO3 + H2S
H2S + 2HNO3 2H2O + 2NO2 + S
2H2O + 2NO2 + S
Na2SO3 + 2HNO3 2NaNO3 + H2O + SO2
2NaNO3 + H2O + SO2
NaHSO3+ HNO3 NaNO3 + H2O + SO2
NaNO3 + H2O + SO2
KOH + HNO3 KNO3 + H2O
KNO3 + H2O
Uses
- In the manufacture of nitrates which are important chemicals of commerce. Basic calcium nitrate is used as a fertilizer. Silver nitrate is used in photography and sodium nitrate in the manufacture of gun powder and fireworks.
- In the manufacture of explosives like nitroglycerine, dynamite, trinitrotoluene (TNT) picric acid etc.
- In the manufacture of artificial silk, dyes medicines and perfumes.
- In the purification of gold and silver.
- As an important reagent in the laboratory.
- In the manufacture of H2SO4
- In etching designs on wares of brass, bronze, etc.
- In the preparation of aqua regia.
