
Ferrous Sulphate
Inorganic Compound of Class 12
Ferrous Sulphate
This is the most important ferrous salt. It is also called green vitriol. We also call it as copperas because it is a by product of the hydrometallurgy of copper. In the laboratory it is prepared: (i)By dissolving iron in dil. H2SO4filtering, evaporating and crystallizing.
Fe + H2SO4 → FeSO4+ H2
(ii)From Kipp’s waste: Kipp’s waste (or residue) contains FeSO4along with free H2SO4. The waste is heated with a small quantity of scrap iron which reacts with excess of H2S to give FeSO4and nascent hydrogen. This nascent hydrogen reduces any ferric iron, if present, to ferrous state. On allowing the hot solution to stand, crystals of FeSO4.7H2O separate out. On a large scale it is obtained by the slow oxidation of iron pyrites in the presence of air and moisture. The pyrites are exposed to air in big heaps and the following reaction takes place.
2FeS2 + 2H2O + 7O2 → 2FeSO4+ 2H2SO4
The free H2SO4is removed by the addition of scrap iron, which also reduces any ferric sulphate formed. On crystallization we get green coloured crystals containing seven molecules of water of crystallization. These crystals are monoclinic and are isomorphous with MgSO4.7H2O. Ferrous sulphate containing 6, 5, 3, 2, 1 molecules and no water of crystallization is also known.
Properties
- Ferrous sulphate forms pale green monoclinic crystals of heptahydrate, FeSO4.7H2O which is isomorphous with the corresponding heptahydrates of cobalt, nickel, chromium, manganese, magnesium, zinc, etc i.e. with MSO4.7H2O where M = Co, Ni, Cr, Mn, Mg, Zn etc. Crystalline hydrates having 6, 5, 4, 3 and 2 water molecules are also known. FeSO4.5H2O is isomorphous with blue vitriol, CuSO4.5H2O.Anhydrous salt (i.e. FeSO) is white.
-
Action of heat: When heated to redness, FeSO4 and FeSO4.7HO both decompose giving a residue of Fewhich is used as a pigment under the name of rogue or venetian red.
2FeSO4 Fe2O3+ SO2 + SO3
Fe2O3+ SO2 + SO3
2[FeSO4.7H2O] Fe2O3 + H2 SO4 + SO2 + 13H2O
Fe2O3 + H2 SO4 + SO2 + 13H2O -
Reducing Properties: It is an active reducing agent, e.g.
It decolorizes acidified KMnO4 solution
2KMnO4+ 10FeSO4 + 8H2SO4 → K2SO4+ 2MnSO4+ 5Fe2(SO4)3 + 8H2O
It turns acidified K2Cr2O7 green
→ K2SO4 + Cr2(SO4)3 + 3Fe(SO4)3 + 7H2O
It reduces NO2 to NO which then gives nitrosoferrous sulphate, Fe(NO)SO4
2FeSO4 + H2SO4 + → Fe2(SO4)3 + H2O + NO
FeSO4 + NO Fe(NO)SO4
Fe(NO)SO4
Nitrosoferrous sulphate
It reduces silver and gold salts to metal.
Ag+ + Fe2+ → Ag↓ + Fe3+
Au3+ + 3Fe2+ → Au↓ + 3Fe3+
It reduces HgCl2 + 2Fe2+ → Hg2Cl2 + 2Fe3+ + 2Cl− -
Formation of addition compound with NO: A cold solution of ferrous sulphate absorbs NO forming dark brown nitroso ferrous sulphate. Fe(NO)SO4 which is destroyed by the action of heat.
FeSO4NO Fe(NO)SO4
Fe(NO)SO4
Nitroso ferrous sulphate -
Action of air: When exposed to air, the green crystals of FeSO4effloresce and turn brown due to formation of basic ferric sulphate, Fe(OH)SO4. Its formation is due to the surface oxidation of FeSO4 to Fe(OH)SO4.
4FeSO4 + 2H2O + O 2 → 4Fe(OH)SO4
GreenBrown -
Formation of double salts: With sulphates of alkali metals it readily forms double salts with the composition, R2SO4.FeSO4.6H2O where R = an alkali metal or
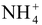 radical. Out of these double salts, ferrous ammonium sulphate (called Mohr’s salt), (NH4)2SO4.FeSO4.6H2O is of great use.
radical. Out of these double salts, ferrous ammonium sulphate (called Mohr’s salt), (NH4)2SO4.FeSO4.6H2O is of great use. -
Acid character: It is readily soluble in H2O, the solution is acidic in nature due to its hydrolysis
FeSO4 + 2H2O Fe(OH)2 + H2SO4
Fe(OH)2 + H2SO4
The solution readily passes into ferric state by atmospheric oxidation.
4FeSO42H2SO 4 + O2 → 2Fe(SO4)3 + 2H2O -
Action of KCN: When excess of KCN is added to ferrous sulphate solution, potassium ferrocyanide, K4[Fe(CN)6] is formed.
FeSO4 + 2KCN → Fe(CN)2+ K2SO4
Brown ppt.
Fe(CN)2 + 4KCN → K4[Fe(CN)6]
——————————————————
FeSO4+ 6KCN → K4[Fe(CN)6] + K2SO4
Uses
- In the manufacture of writing ink, ferrous ammonium sulphate (Mohr’s salt), iron alum. ferric oxide (rouge) etc.
- As a mordant in dyeing
- As a weed−killer in agriculture.
- As a reducing agent.
- As a reagent in the laboratory.
- For clarifying water.
