
Calcium Hydroxide
Inorganic Compound of Class 12
Preparation
(i) From quick lime: Calcium hydroxide is prepared on a commercial scale by adding water to quick lime.
CaO + H2O  Ca(OH)2
Ca(OH)2
(ii) From calcium chloride: It is also obtained by treating calcium chloride with caustic soda.
CaCl2 + 2NaOH  Ca(OH)2 + 2NaCl
Ca(OH)2 + 2NaCl
Properties
(i) It is a white amorphous powder sparingly soluble in water, the solubility decreases further with rise in temperature. An aqueous suspension of calcium hydroxide in water is called milk of lime.
(ii) Reaction with chlorine: It reacts with chlorine to form bleaching powder.
Ca(OH)2 + Cl2  CaOCl2 + H2O
CaOCl2 + H2O
Slaked lime Bleaching powder
(iii) Reaction with carbon dioxide: When carbon dioxide is passed through lime water, it turns milky due to the formation of insoluble calcium carbonate.
Ca(OH)2 + CO2  CaCO3↓ + H2O
CaCO3↓ + H2O
(Milkiness)
On passing excess of carbon dioxide, the precipitate of calcium carbonate dissolve to form soluble calcium bicarbonate and hence the milkiness disappears.
CaCO3 + CO2 + H2O  Ca(HCO3)2
Ca(HCO3)2
Soluble
If this clear solution of calcium bicarbonate is heated, the solution again turns milky due to the decomposition of calcium bicarbonate back to calcium carbonate.
Ca(HCO3)2(aq) 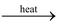 CaCO3(s) + CO2(g) + H2O(l)
CaCO3(s) + CO2(g) + H2O(l)
(iv) Reaction with acids: Slaked lime being a strong base reacts with acids forming salts.
Ca(OH)2 + 2HCl  CaCl2 + 2H2O
CaCl2 + 2H2O
However, Ca(OH)2 does not dissolve in dil. H2SO4 because the calcium sulphate formed is insoluble in water.
Uses
(i) Calcium hydroxide is used as a building material, mortar.
(ii) It is used for softening of hard water.
(iii) In the laboratory, it is used as lime water for detection of carbon dioxide.
(iv) It is also used in making glass, as a cheap alkali for neutralizing acids, to liberate ammonia from ammonia salts and in the purification of sugar and coal gas.
