
Phosphorus
Inorganic Compound of Class 12
Occurrence
Phosphorus is a very reactive element and hence it does not occur free in nature. It occurs mainly in the form of phosphate minerals in the crust of the earth. Some important minerals of phosphorus are
- Phosphorite, Ca3(PO4)2
- Fluorapatite, Ca5(PO4)3F or 3Ca3(PO4)2.CaF2
- Hydroxyapatite, Ca5(PO4)3OH or 3Ca3(PO4)2.Ca(OH)2
- Chlorapatite, Ca5(PO4)3CI or 3Ca3(PO4)2.CaCl2
Preparation
Phosphorus is isolated by heating Ca3(PO4)2with coke and silica in an electric furnace at 1770 K. The reactions taking place may be represented as under
2Ca3(PO4)2 + 6SiO2![]() 6CaSiO3 + P4O10
6CaSiO3 + P4O10
P4O10 + 10C ![]() P4 + 10CO
P4 + 10CO
The vapours of phosphorus thus obtained upon condensation give white phosphorus which exists as P4 molecules.
OXIDES AND OXYACIDS OF PHOSPHORUS
Oxides
Two important oxides of phosphorus are
Phosphorus trioxide - P4O6,also called phosphorus oxide or phosphorus (III) oxide
Phosphorus pentoxide -P4O10,also called as phosphoric oxide or phosphorus (V) oxide
P4O6 Preparation
Prepared by burning white phosphorus in limited supply of air
P4 + 3O2 → P4O6
Properties
-
On heating in air, it forms phosphorus (V) oxide
P4O6 + 2O2→P4O10 -
Reacts with water as follows :
P4O6 + 6H2O(cold)→ 4H3PO3
Phosphorus acid
P4O6 + 6H2O(hot)→ 3H3PO4 + PH3
(orthophosphoric acid)
P4O10 Preparation
Prepared by burning white phosphorus in an excess of air or oxygen
P4 + 5O2→ P4O10
Properties
-
Because of its great affinity for water, it acts as a dehydrating agent.
2HNO3 + P4O10 → 2N5O5 + 4HPO3
2H2SO4 + P4O10 → 2SO3 + 4HPO3 -
Reacts with water as follows :
P4O10 + 2H2O(cold) → 4HPO3
P4O10 + 6H2O(hot) → 4H3PO4
Structure of P4O6 and P4O10
|
|
|
Oxyacids
Phosphorus forms two series of oxyacides:
- The phosphoric series of acids, in which the oxidation state of P is +5 or +4 and have oxidizing properties.
- The phosphorous series of acids, which contain P in the oxidation state +1 or +3 and are reducing agents.
Phosphorus forms numerous oxy-acids, all of which contain tetrahedral co-ordinated phosphorus containing at least one P = 0 unit and one P − OH group. The condensed systems are formed by P − O − P links or by direct P − P links.
| Name | Formula | Oxidation State of Phosphorus | Basicity |
| Hypophosphorus acid | H3PO2 | +1 | 1 |
| Phosphorus acid | H3PO3 | +3 | 2 |
| Orthophosphoric acid | H3PO4 | +5 | 3 |
| Hypophosphoric acid | H4P2O6 | +4 | 4 |
| Pyrophosphoric acid | H4P2O7 | +5 | 4 |
| Metaphosphoric acid | (HPO3)n |
+5
|
1 |
Preparation
-
Phosphorus acid (H3PO3)
Prepared by the hydrolysis of phosphorus trichloride or phosphorus trioxide.
PCl3 + 3H2O → H3PO3 + 3HCl
P4O6 + 6H2O → 4H3PO3 -
Orthophosphoric acid (H3PO4)
(a)Prepared by burning phosphorus followed by the hydrolysis of the P4O10 so formed
PO4 + 5O2 → P4O10→ 4H3PO4
(b)By heating phosphate rock with sulphuric acid
Ca3(PO4)2 + 3H2SO4→ 3CaSO4 + 2H3PO4
On heating, it forms pyrophosphoric acid and then metaphosphoric acid on further heating.
2H3PO4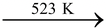 H4P2O7 + H2O
H4P2O7 + H2O
Pyrophosphoric acid
H3PO4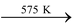 HPO3 + H2O
HPO3 + H2O
Meta phosphoric acid -
Metaphosphoric acid (HPO3):
Prepared by heating orthophosphoric acid to 575K
H3PO4 HPO3 + H2O
HPO3 + H2O -
Pyrophosphoric acid (H4P2O7):
Prepared by heating orthophosphoric acid to about 525 K.
2H3PO4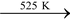 H4P2O7 + H2O.
H4P2O7 + H2O.
Structure of Oxyacids of Phosphorus
|
|
|
|
|
|
|
Hypophosphoric acid, (H4P2O6) |
Phosphorus acid, H3PO3 |
