
Nitrogen
Inorganic Compound of Class 12
Preparation
Nitrogen is prepared by heating ammonium nitrite.
NH4NO2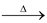 N2↑ + 2H2O
N2↑ + 2H2O
Properties
- Colourless tasteless, odourless neutral gas.
- Highly inert
- Neither combustible nor supporter of combustion however a burning piece to Mg continues to burn in nitrogen. The product formed on hydrolysis gives NH3.
3Mg + N2 Mg3N2↓
Mg3N2↓
Mg3N2 + 6H2O  3Mg(OH)2 + 2NH3↑
3Mg(OH)2 + 2NH3↑
Uses
- Used in the manufacture of ammonia nitric acid, cynamide etc.
- Nitrogen filled thermometers are used for measuring high temperature.
- Liquid N2is used as a refrigerant to preserve biological materials.
Compounds of Nitrogen
Oxyacids
The most important oxo-acid of nitrogen is nitric acid HNO3
Preparation
-
In the lab, it is prepared by heating NaNO3 or KNO3with conc. sulphuric acid in a glass retort.
NaNO3 + H2SO4 →NaHSO4 + HNO3 -
It is manufactured by the catalytic oxidation of ammonia and the process is known as Ostwald Process
4NH3 + 5O2 4NO + 6H2O
2NO + O22NO2
3NO2 + H2O → 2HNO3 + NO.
Properties
- In aq. solution, nitric acid is a strong acid and dissociates to give hydronium and nitrate ions. HNO3 + H2O H3O+ + NO3-
-
Action on metals: Conc. nitric acid is a strong oxidising agent and attacks most of the metals except noble metals such as gold and platinum. The product of reaction depends upon the concentration of the acid, temp and the nature of the material undergoing oxidation. With dilute nitric acid the principle product is nitric oxide NO and with conc. nitric acid, the principle product is N(IV) oxides 3Cu + 8HNO3 → 3Cu(NO3)2 + 2NO + 4H2O (dilute)
Zinc, which is a more powerful reducing agent than copper, reacts with dilute nitric acid to give ammonium nitrate.
4Zn + 10HNO3 → 4Zn(NO3)2 + NH4NO3 + 3H2O
Nitric acid also oxidises non - metals and their compounds. Iodine is oxidised to iodic acid, carbon to carbon dioxide, sulphur to H2SO3 and phosphorus to phosphoric acid. Nitric acid is reduced to nitrogen dioxide (NO2).
I + 10HNO3 → 2HIO3 10NO2 + 4H2O
C + 4HNO3 → CO2 + 2H2O + 4NO2
1/8 S8 + 6HNO3 → H2SO4 + 6NO2 + 2H2O
P4 + 20HNO3 → 4 H3PO4 + 20NO2 + 4H2O
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.