
Hydrogen Peroxide
Inorganic Compound of Class 12
(i) Preparation
(a) Na2O2 + dil. H2SO4  H2O2 + Na2SO4
H2O2 + Na2SO4
(b) BaO2 + H2SO4  H2O2 + BaSO4↓
H2O2 + BaSO4↓
(c) 3BaO2 + 2H3PO4  3H2O2 + Ba3(PO4)2↓
3H2O2 + Ba3(PO4)2↓
(d) BaO2 + H2O + CO2 H2O2 + BaCO3
H2O2 + BaCO3
(ii) By electrolysis of Ammonium hydrogen sulphate (NH4HSO4) in presence of excess of H2SO4
(a) NH4HSO4 → NH4SO-4 + H+
at anode
2NH4SO-4 → (NH4)2S2O8 + 2e−
Ammonium perdisulphate
at cathode:−
2H+ + 2e− → H2↑
On heating Ammonium perdisulphate in presence of water hydrolysis occurs and H2O2 is produced.
(NH4)2S2O8 + 2H2O 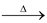 H2O2 + NH4HSO4
H2O2 + NH4HSO4
(iii) Auto oxidation of 2−ethyl or Butyl Anthraquinone
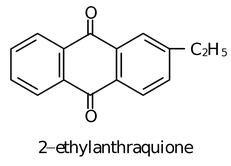
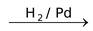
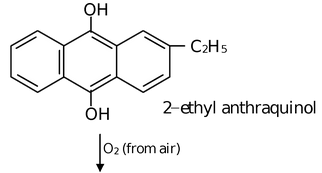
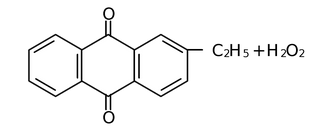
The aqueous product contains 20% H2O2.
PHYSICAL PROPERTIES
(1) Pale blue colour, diamagnetic, surupy liquid.
(2) Unstable in presence of sunlight.
(3) Have high dielectric constant and therefore its aqueous solution is very good solvent for ionic compounds.
CHEMICAL PROPERTIES
(1) Unstable in presence of sunlight
2H2O2(l) 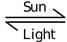 2H2O (l) + O2↑
2H2O (l) + O2↑
(2) Acidic nature: Weak acid
H2O2 → H+ +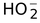
It turns blue litmus red, decomposes carbnonates to CO2 and forms salts with bases.
(3) Oxidising and reducing behaviour:
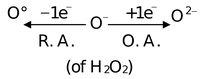
Acts as both oxidizing agent and reducing agent because here oxygen has intermediate oxidation state of '−1' in H2O2.
(a) H2O2 as an oxidising agent:
In acidic and basic both medium H2O2 behaves as a very good oxidizing agent.
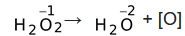
O.A.
It oxidising power can be understood better by
H2O2 + 2H+ + 2e- → 2H2O E° = 1.77 V
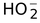 + H2O + 2e- → 3OH- E° = 0.87 V
+ H2O + 2e- → 3OH- E° = 0.87 V
e.g. (i) PbS↓ + 4H2O2 → PbSO4↓ + 4H2O
Black white
[H2O2 → H2O + [O] ] × 4
PbS + 4[O] → PbSO4
—————————————————
PbS + 4H2O2 → PbSO4 + 4H2O
—————————————————
Therefore old oil painting rendered black by atmospheric H2S can be whitened with H2O2.
(ii) Na2SO3 + H2O2 → Na2SO4 + H2O
(iii) KNO2 + H2O2 → KNO3 + H2O
(iv) Fe2++ 2H++ H2O2 → Fe3+ + 2H2O
Light green yellow
2FeSO4 + H2SO4 + H2O2 → Fe2(SO4)3 + 2H2O
(v) 2K4[Fe(CN)6] + H2O2 → K3[Fe(CN)6] + KOH
Ferrocyanide Ferriccyanide
(vi) 2I− + 2H+ + H2O2 → I2↑ + 2H2O
(vii) With acidic K2Cr2O7, deep blue colour of CrO5 is obtained which have greater stability in ether.
Cr2O-24+ H2O2 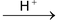 CrO5 + H2O
CrO5 + H2O
(b) H2O2 as Reducing agent:
H2O2 → O2 + 2[H]
e.g. (i) O3 + H2O2 → 2O2 + H2O
(ii) Cl2 + H2O2 → 2HCl↑ + O2↑
(iii) Decolourises violet colour of acidic KMnO4.
2KMnO4 + 3H2SO4 + H2O2 → 2MnSO4 + K2SO4 + 8H2O + 5O2↑
Violet almost colourless
In alkaline solution MnO2 is formed.
2KMnO4 + 3H2O2 → 2MnO2↓ + 3O2 + 2H2O + 2KOH
Brown
(iv) 2K3[Fe(CN)6] + 2KOH + H2O2 → 2K4 [Fe(CN)6] + 2H2O + O2↑
(v) Ag2O + H2O2 → 2Ag + H2O + O2↑
(iv) Bleaching Action
Causes permanent bleaching as it occurs due to oxidising behaviour of H2O2.
H2O2 → H2O + [O]
Colour + [O] → Colourless
Test of H2O2
(i) It gives blue ethereal layer with acidic K2Cr2O7 having some ether.
K2Cr2O7 + H2SO4 + H2O2 → CrO5 + K2SO4 + H2O
forms blue
ethereal layer.
(ii) It gives Iodine with KI which turns starch paper blue.
2KI + H2O2 → 2KOH + I2
Starch + I2 → Blue Starch Iodine Complex
(iii) Forms orangish yellow solution with acidic TiO2
TiO2 + H2SO4 + H2O2 → orangish red or yellow (when dilute).
TiO2.nH2O or H2[TiO2(SO4)2]
pertitanic acid. peroxo−disulphate titanic acid
Note: The Concentration of H2O2 solution is measured in terms of volume strength, which you have learnt already in stoichiometry. 30% solution of H2O2 is sold in market under the trade name perhydrol.
