Aluminium Chloride
Inorganic Compound of Class 12
Preparation
When aluminium metal or Al(OH)3 is treated with HCl, solution of hydrated aluminium chloride, AlCl3.6H2O is obtained.
2Al + 6HCl  2AlCl3 + 3H2↑
2AlCl3 + 3H2↑
Al(OH)3 + 3HCl  AlCl3 + 3H2O
AlCl3 + 3H2O
On evaporating the solution, the crystals of AlCl3.6H2O are obtained. On heating these crystals, anhydrous aluminium chloride cannot be obtained due to the formation of Al2O3.
2(AlCl3.6H2O)  Al2O3 + 6HCl + 9H2O
Al2O3 + 6HCl + 9H2O
The anhydrous aluminium chloride is however prepared as follows:
(i) By passing dry chlorine gas or vapours of hydrochloric acid gas over heated aluminium powder. The vapour of aluminium chloride is condensed in the receiver.
2Al + 3Cl2  2AlCl3
2AlCl3
2Al + 6HCl  2AlCl3 + 3H2↑
2AlCl3 + 3H2↑
(ii) By heating a mixture of alumina and coke in a current of chlorine (Mac Affe process)
Al2O3 + 3C + 3Cl2  2AlCl3 + 3CO
2AlCl3 + 3CO
(iii) By heating alumina (Al2O3) in a current of carbonyl chloride (COCl2).
Al2O3 + 3COCl2  2AlCl3 + 3CO2
2AlCl3 + 3CO2
(iv) By heating alumina in a current of S2Cl2 vapour and Cl2. (Commercial preparation)
4Al2O3 + 3S2Cl2 + 9Cl2  8AlCl3 + 6SO2
8AlCl3 + 6SO2
Properties
(i) Anhydrous salt is a white crystalline solid. It is hygroscopic and fumes in moist air. It sublimes at 180°C. (Melting point = 193°C at 2 atm pressure).
(ii) It is a typical covalent compound as is shown by its volatility, solubility in organic solvents like C6H6, CS2 etc and poor conductivity of the fused state.
(iii) An aqueous solution of the salt is acidic in nature because in water it undergoes hydrolysis and forms HCl.
AlCl3 + 3H2O  Al(OH)3 + 3HCl
Al(OH)3 + 3HCl
With moist air, the salt gives the fumes of HCl. The equation representing the reaction is the same as given above.
(iv) When NH4OH is added to AlCl3 solution, white precipitate of Al(OH)3 is obtained.
AlCl3 + 3NH4OH  Al(OH)3↓ + 3NH4Cl
Al(OH)3↓ + 3NH4Cl
White
(v) With excess of NaOH, the solution of the salt gives sodium meta−aluminate, NaAlO2.
AlCl3 + 4NaOH (excess)  NaAlO2 + 2H2O + 3NaCl
NaAlO2 + 2H2O + 3NaCl
(soluble)
(vi) It forms addition compounds with a number of donor molecules like NH3, PH3,
COCl2 etc.
AlCl3 + 6NH3  AlCl3.6NH3
AlCl3.6NH3
Addition compound
Uses
(i) In organic chemistry for introducing alkyl radicals originally linked with halogen into benzene nucleus (Friedel Craft’s reaction).
(ii) In the manufacture of petrol by the cracking of mineral oils.
(iii) As a catalyst in the manufacture of dyes, drugs and perfumes.
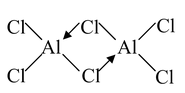
Dimerization of AlCl3
2AlCl3  Al2Cl6
Al2Cl6
|
The dimmer has the structure shown in the diagram. In this structure, the halogen atoms are tetrahedrally arranged about each aluminium atom. The dimeric formula is retained when it is dissolved in non−polar solvents such as benzene, but because of higher heat of hydration, the covalent dimer is broken into [Al.6H2O]3+ and 3Cl− ions on dissolution in water. |
|