
Magnesium Sulphate
Inorganic Compound of Class 12
It occurs in nature as minerals kiesserite (MgSO4.H2O), Epsom salt (MgSO4.7H2O) and kainite (KCl.MgSO4.3H2O).
Preparation
It is formed by reacting magnesite (MgCO3) or dolomite with dilute sulphuric acid.
MgCO3 + H2SO4 → MgSO4 + H2O + CO2
MgCO3.CaCO3 + 2H2SO4 → MgSO4 + CaSO4 + 2CO2 + 2H2O
Dolomite (Insoluble)
It is commercially prepared by boiling kieserite mineral in water. The crystals are obtained when the solution is cooled.
MgSO4.H2O + 6H2O → MgSO4.7H2O
Properties
It is a colourless crystalline compound, soluble in water. The crystals are efflorescent and bitter in taste. It is isomorphous with ZnSO4.7H2O. It forms double sulphates with alkali metal sulphates, K2SO4.MgSO4.6H2O (Schonite).
Heating effect: When heated to 150°C, it changes to monohydrate. On further heating, it becomes anhydrous at 200°C. On strong heating, it decomposes into MgO.
MgSO4.7H2O 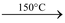 MgSO4.H2O
MgSO4.H2O 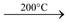 MgSO4
MgSO4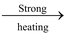 MgO + SO2 + ½O2
MgO + SO2 + ½O2
Magnesium sulphate is reduced by lampblack at 800°C.
2MgSO4 + C → 2MgO + 2SO2 + CO2
Uses
(i) as a purgative in medicine.
(ii) as a filler for paper.
(iii) as a mordant in dyeing and tanning industry.
(iv) in the manufacture of paints and soaps and in fire−proofing fabrics.
