
The Noble Gases
Inorganic Compound of Class 12
The Noble Gases
There are six monoatomic gases (viz. He, Ne, Ar, Kr, Xe, Rn) placed in group−18 called noble gases. All these gases are colourless, tasteless and odourless and exist in the atmosphere. They have the most stable configuration of the type ns2np6(except He for which it is 1s2). So noble gas atom have almost zero electron affinity and very high ionization potential than any other element. Hence they do not lose or gain electrons under normal conditions and do not form bonds. However, Xenon forms chemical compounds with fluorine and oxygen.
The first break through in this regard was made by Bartlett and Lohmann when they synthesized first chemical compound of noble gas, XePtF6 (or, Xe+(PtF6)-, a yellow solid. Xenon reacts with fluorine under varying condition to give XeF2, XeF4 and XeF6.
(i) Xe + F2 ![]() XeF2
XeF2
Product is purified by cooling at −78°C to remove unchanged Xe or F2 and finally by distilling.
(ii) Xe + F2 ( 1 : 5 ratio) ![]() XeF4
XeF4
colourless crystals
Purified as in case of XeF2
(iii) Xe + F2 ( 1: 20 parts) ![]() XeF6
XeF6
colourless crystals
Purified by flash distillation.
The above three fluorides are all crystalline solid at room temperature. The fluoride dissolves in anhydrous hydrogen fluoride to give highly electrical conductive solution as
XeFn + HF 
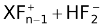
The fluorides are very strong oxidising and fluorinating agents. Such as
XeF2 + H2  Xe + 2HF
Xe + 2HF
(Fluorinating
agent)
XeF2 + 2HCl  Xe + 2HF + Cl2
Xe + 2HF + Cl2
Oxidising agent
Fluorides undergo hydrolysis to finally give a colourless deliquescent solid XeO3. The hydrolysis takes place in successive steps. In each step a pair of fluoride ion is substituted by one oxygen atom.
XeF6 + H2O  XeOF4 + 2HF
XeOF4 + 2HF
XeOF4 + H2O  XeO2F2 + 2HF
XeO2F2 + 2HF
XeO2F2 + H2O  XeO3 + 2HF
XeO3 + 2HF
